Konference: 2015 57th ASH Annual Meeting - účast ČR
Kategorie: Mnohočetný myelom
Téma: 653. Myeloma: Therapy, excluding Transplantation: Poster III
Číslo abstraktu: 4250
Autoři: MD Stuart D. Russell; Dr. Alexander Lyon, BHF; MD Daniel J. Lenihan; Prof. MD Philippe Moreau; Douglas E. Joshua; Wee Joo Chng; MD Antonio P. Palumbo; Prof. Dr. med. Hartmut Goldschmidt; prof. MUDr. Roman Hájek, CSc.; MD Thierry Facon; Prof. MD Heinz Ludwig; prof. MUDr. Luděk Pour, Ph.D.; MD Ruben Niesvizky; M.D. Albert Oriol; MD Laura Rosiñol, PhD; M.D. Alexander Suvorov; MD Gianluca Gaidano, PhD; MD Vesselina Goranova-Marinova, PhD; Heidi H. Gillenwater; Nehal Mohamed; Shibao Feng; Meletios Athanasios Dimopoulos, MD
Introduction: The ENDEAVOR study found that Kd was superior to Vd, with a 2-fold improvement in median PFS (18.7 vs 9.4 mo; HR=0.53; 95% CI, 0.44–0.65; P<.0001; Dimopoulos et al, Haematologica 2015;100[s1]:abst LB2071). A substudy was conducted within ENDEAVOR to evaluate left and right ventricular function via echocardiogram (ECHO) in a subset of enrolled pts.
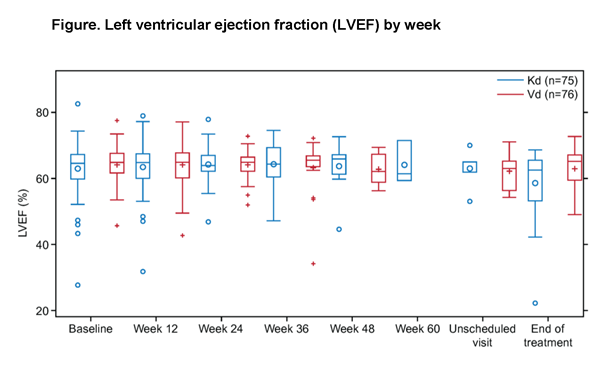
Methods: Adults with RMM (1–3 prior regimens) were eligible. Pts must have had left ventricular ejection fraction (LVEF) ≥40% and could not have New York Heart Association class III or IV symptomatic heart failure (HF), symptomatic ischemia, uncontrolled arrhythmias or recent myocardial infarction within 4 mo before randomization. Pts were randomized 1:1. The Kd arm received K (20/56 mg/m2; 30‑min IV infusion on days [D] 1, 2, 8, 9, 15, 16 [20 mg/m2 on D1, 2 of cycle 1; 56 mg/m2 thereafter]) and dexamethasone 20 mg on D1, 2, 8, 9, 15, 16, 22, 23 (28-day cycle). The Vd arm received V (1.3 mg/m2; IV or SC) on D1, 4, 8, 11 and dexamethasone 20 mg on D1, 2, 4, 5, 8, 9, 11, 12 (21-day cycle). All pts at qualified centers were enrolled in the substudy and assessed with 2D transthoracic ECHO at baseline (BL), every 12 wk on D1 of treatment cycles, and the end-of-treatment visit. ECHO images were read centrally and interpreted for clinical significance by an independent cardiac events adjudication committee (CEAC).
The substudy objective was to assess change from BL in LVEF, right ventricular (RV) function, and pulmonary artery systolic pressure (PASP). The prespecified primary endpoint assessed whether there was a significant reduction (≥10% absolute decrease from BL in pts with LVEF ≤55% or a reduction to <45% for pts with LVEF >55% at BL) or no change in LVEF within 24 wk from BL. A mixed model for repeated measures (MMRM) under the assumption of missing-at-random was used to estimate longitudinal differences in the absolute score (continuous) between treatment arms.
Results: Of 929 pts overall, 151 (Kd: 75; Vd: 76) enrolled in the substudy; 74 in each arm were evaluable for safety. In the substudy, more Kd vs Vd pts were aged ≥75 yr (21.3% vs 14.5%), had prior cardiac-related medical history (26.7% vs 14.5%), and received drugs for obstructive airway disorders (28.0% vs 17.1%).
Kd pts had a higher incidence of HF reported as an AE vs Vd pts (10.8% [n=8] vs 4.1% [n=3]), consistent with the overall safety population (8.2% vs 2.9%). History of cardiac disorders was associated with an elevated (but not statistically significant) risk of HF (3/8 Kd pts; 0/3 Vd pts). Kd vs Vd pts had a higher incidence of hypertension (HTN; substudy: 20.3% vs 8.1%; overall: 24.8% vs 8.8%).Treatment was discontinued due to AEs in 23 pts (15.2%); 8 due to cardiac-related AEs (6 Kd [2 due to LV dysfunction, and 1 each due to HF, decreased EF, pulmonary embolism, and ischemic stroke] and 2 Vd [1 each due to decreased EF and RV failure]). No substudy pts had a fatal cardiac AE.
For the primary endpoint, only 1 pt (Vd) had significant LVEF reduction as defined above within the first 24 wk of study. Three additional pts had a significant LVEF reduction at any time during the study (Kd: 2; Vd: 1). All but 1 pt had resolution to normal LVEF.
MMRM analysis of LVEF reduction and RV function found no significant treatment or treatment-by-time interactions, and no significant difference between the Kd and Vd groups at any time point (P-values ranged from 0.07 to 0.91). MMRM analysis of PASP was inconclusive, with significant PASP increase over time in the Vd (but not Kd) arm, but also a significant difference in PASP in the Kd vs Vd arms (28.38 vs 24.06 mmHg; P=0.02) at wk 12 only. However, the difference between arms decreased over time and was not significant at later time points.
CEAC evaluation found 14 pts (Kd: 8; Vd: 6) had clinically meaningful changes in ECHOs while on study; 11/14 (79%) did not meet the ECHO criteria for decreased LVEF as defined earlier. The CEAC reported that more Kd vs Vd pts had evidence of HF (n=4 vs n=0) or pulmonary HTN (n=4 vs n=1).
Conclusions:
ENDEAVOR was the first K study to require BL assessment of LVEF or
serial ECHO assessments over time. In the overall study and cardiac
substudy, HF and pulmonary HTN events were more frequently reported
in Kd vs Vd pts. LVEF reductions were mostly reversible. The
substudy found limited utility for serial screening with ECHOs as a
risk mitigation tool for unselected pts receiving K. Improved
surveillance strategies are needed to detect early cardiotoxicity
and prevent treatment interruption.
Disclosures: Lyon: Onyx Pharmaceuticals: Consultancy . Moreau: Novartis, Janssen, Celgene, Millennium, Onyx Pharmaceuticals: Consultancy , Honoraria . Joshua: Celgene: Membership on an entity’s Board of Directors or advisory committees . Palumbo: Amgen, BMS, Genmab A/S, Celgene, Janssen-Cilag, Millennium Pharmaceuticals, Inc, Onyx Pharmaceuticals, Sanofi Aventis, Array BioPharma: Consultancy , Honoraria . Goldschmidt: Chugai, Millennium: Honoraria , Research Funding ; BMS: Consultancy , Research Funding ; Amgen, Takeda: Consultancy ;Onyx: Consultancy , Honoraria ; Janssen, Celgene, Novartis: Consultancy , Honoraria , Research Funding . Hájek:Janssen-Cilag: Honoraria ; Celgene, Amgen: Consultancy , Honoraria . Facon: Onyx/Amgen: Membership on an entity’s Board of Directors or advisory committees . Ludwig: Takeda, Onyx, Bristol Meyers, Celgene, Janssen Cilag:Honoraria , Research Funding , Speakers Bureau . Niesvizky: Celgene, Millennium, Onyx: Consultancy , Speakers Bureau . Gaidano: Roche: Honoraria , Other: Advisory boards ; Morphosys: Honoraria , Other: Advisory boards ;Novartis: Honoraria , Other: Advisory boards ; GlaxoSmithKline: Honoraria , Other: Advisory boards ; Amgen:Honoraria , Other: Advisory boards ; Janssen: Honoraria , Other: Advisory boards ; Karyopharm: Honoraria , Other: Advisory boards ; Celgene: Research Funding . Gillenwater: Onyx, Amgen: Employment , Other: Stock . Mohamed:Onyx, Amgen: Employment , Other: Stock . Feng: Amgen/Onyx: Employment , Equity Ownership . Dimopoulos:Janssen-Cilag: Honoraria ; Genesis: Honoraria ; Novartis: Honoraria ; Amgen: Honoraria ; Janssen: Honoraria ;Onyx: Honoraria ; Celgene: Honoraria .
Datum přednesení příspěvku: 7. 12. 2015