Konference: 2006 48th ASH Annual Meeting - účast ČR
Kategorie:
Maligní lymfomy a leukémie
Téma: Simultaneous session: Chronic Lymphotic Leukemia (CLL) Therapy: Novel Clinical Approaches
Číslo abstraktu: 301
Autoři: Peter Hillmen, M.B. ChB, Ph.D.; Aleksander Skotnicki; MD Tadeusz Robak, PhD.; Branimir Jaksic; Cynthia Sirard; prof. MUDr. Jiří Mayer, CSc.
CAM307 is a phase III, open-label, multinational, randomized
controlled trial comparing alemtuzumab (CAM) with chlorambucil
(CHLO) for previously untreated BCLL requiring therapy. Eligible
patients with Rai Stages I-IV were randomized 1:1 to either CAM 30
mg IV tiw for up to 12 weeks or CHLO 40 mg/m
2 po q 28
days up to 12 cycles. CAM patients received prophylactic
trimethoprim/sulfamethoxazole DS and famciclovir treatment during
therapy and until CD4
+ counts were ≥200 cells/µL. The
primary endpoint was PFS; secondary endpoints were response rate,
overall survival, and safety. A total of 297 patients were enrolled
(CAM n=149 and CHLO n=148); median age: 60 years; performance
status 0-1: 96%; maximum lymph node size ≥5 cm: 22%; and Rai Stage
I-II: 63%, Rai Stage III-IV: 33%. Diagnosis, Rai Stage, response
and disease progression were confirmed by an independent response
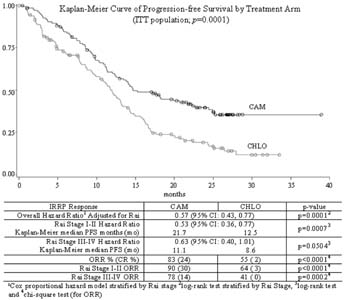
review panel (IRRP). CAM has a significantly prolonged PFS compared
to CHLO (p=0.0001; see KM survival curve).PFS in CAM vs CHLO
patients with adverse cytogenetic findings of del 17p (n=21) was
10.7 mo vs 2.2 mo and for trisomy 12 (n=39) was 18.3 mo vs 12.9 mo.
Results were similar for patients with del 11q (n=54, 8.5 mo vs 8.6
mo). Common (≥15%) CAM reported adverse events (AEs) (n=147),
likely infusion-related, included pyrexia (70%), chills (53%),
nausea (18%), hypotension (16%) and urticaria (16%). Common AEs
(≥15%) in the CHLO arm (n=147) were nausea (37%) and vomiting
(18%). Frequency of asymptomatic CMV viremia on the CAM arm was
52%; CMV infection occurred in only 16%, none grade 4. CAM
treatment was interrupted in 56% of CMV viremic patients, and
resumed in 92% with ORR 92% (31% CR). Ganciclovir was administered
to 41% of CAM patients with CMV viremia. Infections, including CMV,
were reported in 76% of CAM and 50% of CHLO patients while on
study. Relevant grade 3/4 treatment-emergent events included (CAM
vs CHLO): lymphopenia (97% vs 3%), pyrexia (8% vs 0%), CMV events
(8% vs 0%) and chills (3% vs 0%). Treatment emergent grade 3/4
thrombocytopenia (16% vs 13%) and anemia (14% vs 19%) were similar,
and although grade 3/4 neutropenia was more common with CAM, (45%
vs. 26%), AEs of CAM vs CHLO bacteremia/sepsis (3% vs 2%) and
febrile neutropenia (5% vs 3%) were comparable. Serious AEs were
reported for 44% of CAM (20% for IV Ganciclovir only in CMV viremic
patients) and 20% of CHLO patients while on study treatment.
CONCLUSIONS: CAM307 demonstrates that therapy-nave BCLL patients
treated with CAM have significantly longer PFS and higher ORR than
those treated with CHLO, with manageable toxicities. Although CMV
reactivation was reported in the CAM arm, prompt intervention
maintained efficacy. The activity seen here supports ongoing
investigations of CAM in first line combination therapy, high risk
patients and consolidation.
Datum přednesení příspěvku: 11. 12. 2006






