Konference: 2014 19th Congress of the European Hematology Association - účast ČR
Kategorie: Myeloproliferativní nemoci
Téma: Immuno-thrombocytopenia (Poster)
Číslo abstraktu: P601
Autoři: Prof. MD Helen A. Papadaki, PhD; MD Ann Janssens; Georgia Kaiafa; doc. MUDr. Tomáš Kozák, Ph.D., MBA; MD Dominik Selleslag; MD Michael Steurer; Dr. Philippe Quittet; MD Jean-François Viallard, PhD; Prof. MD Hans Wadenvik; Laura (Jane) Belton 1976-; Georg Kreuzbauer
ABSSUB-3206
Background: ITP is characterized by low platelet counts and increased risk of bleeding. Severe bleeding episodes are uncommon but may be life-threatening and require hospitalization. Romiplostim is a thrombopoietin-mimetic peptibody approved in Europe for second line treatment in splenectomised and nonsplenectomised pts where surgery is contraindicated.
Aims: To describe romiplostim use in European clinical practice, and to describe the demographics, clinical outcomes, and resource utilization in romiplostim-treated pts. Results from a planned interim analysis are reported.
Methods: Eligible pts were adults with primary ITP who had received romiplostim and who had provided informed consent. The observation period was for up to 2 years after romiplostim initiation. Data were collected retrospectively on ITP-related hospitalizations for up to 2 years before romiplostim initiation and on bleeding events for up to 6 months before romiplostim initiation.
Results: An interim analysis was conducted in September 2013. In total, 356 pts had enrolled and 339 were included in the Full Analysis Set. The mean (±SD) duration of romiplostim treatment was 21 (±5) months; 25% of pts remained on study, 11% discontinued, and 65% completed the 2-year observation period. Adverse drug reactions (ADRs) led to early romiplostim discontinuation in 5% of pts. At romiplostim initiation, the median age was 62 years (range 18–91) and median duration of ITP was 3 years (range 0–56), with one-third of pts having ITP for less than 1 year. Most pts (55%) had received ≥3 prior ITP therapies and 34% had undergone splenectomy. The median average weekly dose of romiplostim was 2.8 mcg/kg (Q1-Q3: 1.5–4.4) and most pts (70%) started romiplostim at a 1 mcg/kg dose. Some intermittent dosing with romiplostim was observed: 41% of pts had a >3 week period where doses were withheld or missing. Romiplostim was self-administered by 37% of pts. The median baseline platelet count was 20 x 109/L (Q1-Q3: 9-35), which rose to 69 x 109/L (Q1-Q3: 28-139) after 2 weeks of romiplostim treatment and remained >50 x 109/L thereafter. Thirty pts (9%) ended romiplostim treatment due to achieving a haemostatic platelet count range, with median platelet counts of 172 x 109/L (Q1-Q3: 90-261). After romiplostim initiation, there was a decrease in rates of grade ≥3 bleeding events (from 12 to 2 per 100 pt-years) and ITP-related hospitalizations (from 87 to 35 per 100 pt-years). There were some differences in baseline demographics between splenectomised and nonsplenectomised pts, but romiplostim doses and bleeding and hospitalization rates were similar between the two groups (Table). Thirteen pts reported a serious ADR. Seven pts experienced 8 thrombotic ADRs (2 each of deep vein thrombosis and pulmonary embolism; 1 each of embolism, myocardial infarction, retinal vein thrombosis, and transient ischaemic attack). Bone marrow fibrosis ADRs occurred in two pts as reported in previous interim analyses (myelofibrosis in pts who were subsequently found to have a diagnosis inconsistent with ITP [MDS and metastases to bone marrow]). No fatal ADRs were reported.
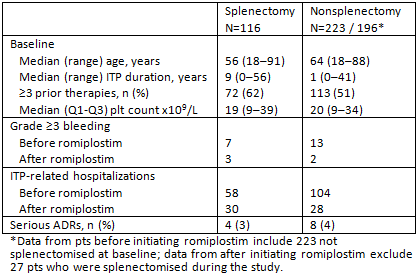
Summary/Conclusion: The dosing, effectiveness, and safety of romiplostim in a real-world ITP pt population appeared comparable with that observed in clinical studies (e.g. Kuter BrJH 2013). Hospitalization and bleeding were lower after romiplostim treatment, and outcomes were similar between splenectomised and nonsplenectomised pts.
Keywords: Bleeding, Immune thrombocytopenia (ITP), Thrombopoietin (TPO)
Datum přednesení příspěvku: 17. 6. 2014





