Konference: 2015 57th ASH Annual Meeting - účast ČR
Kategorie: Ostatní
Téma: 902. Health Services and Outcomes Research – Malignant Diseases: Poster I
Číslo abstraktu: 2096
Autoři: Prof. Dr. med. Christian Buske; Dr. Shalal Sadullah, FRCP, FRCPath; MD Efstathios Kastritis; Giulia Benevolo; Dr. Ramón García Sanz; MD Lukasz Bolkun, Ph.D.; MD Xavier Leleu, PhD; MD Wolfgang Willenbacher; prof. MUDr. Roman Hájek, CSc.; MD Ellen van der Spek, Ph.D.; Mei Cheng, Ph.D.; MD Thorsten Graef, PhD; Meletios Athanasios Dimopoulos, MD
Introduction
Waldenström’s Macroglobulinemia (WM) is a rare indolent lymphoma with a low incidence of ~3 cases per million per year. There are few randomized trials and no well-established treatment standards in WM. Treatment landscapes for treatment-naïve and relapsed WM are heterogeneous and data on treatment choices and their outcome in patients (pts) outside clinical trials are lacking. The goal of this project was to generate data on epidemiologic/treatment patterns and efficacy outcomes for WM over a prolonged period of time (~10 yr) in a large pan-European effort.
Methods
In this observational chart review, physicians completed a retrospective electronic record for pts who fit the following inclusion criteria: confirmed WM, symptomatic disease at treatment initiation, front line treatment initiated between Jan 2000-Jan 2014, and availability of complete clinical/biologic evaluation at diagnosis/initial therapy. Study endpoints included initial/subsequent lines of treatment, progression-free survival (PFS), and overall survival (OS). The number of pt records per country was prespecified to balance the distribution between European countries.
Results
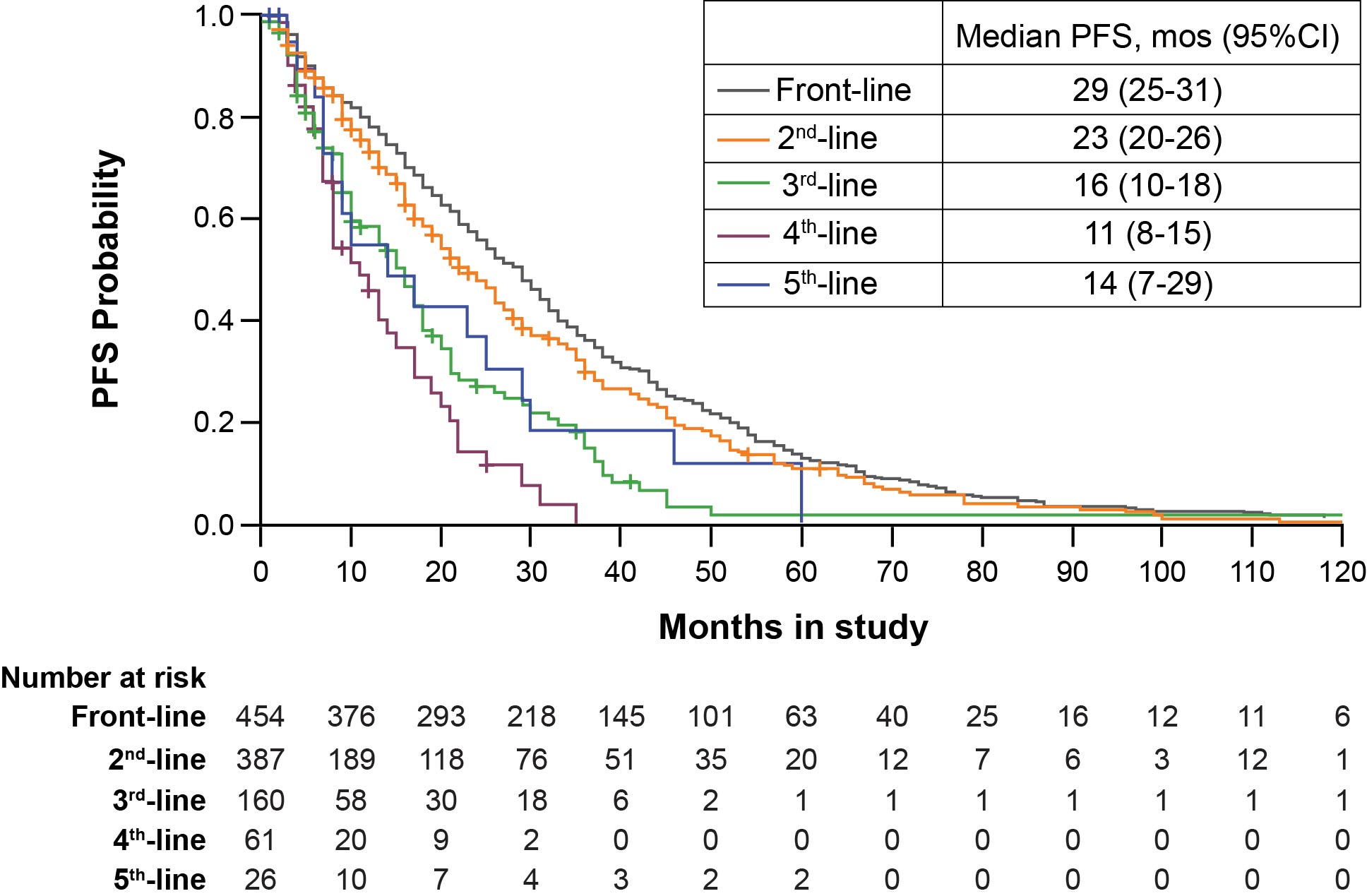
Of 454 pt records reviewed, cases were from France (n=92), United Kingdom (UK; n=72), Germany (n=66), Spain (n=60), Italy (n=56), Greece (n=25), Netherlands (n=25), Poland (n=21), Austria (n=19), and Czech Republic (n=16). Data were summarized across 5 lines of treatment for 454, 397, 160, 61, and 26 pts, respectively. Median age at initiation of front-line treatment was 65 yr (range, 29-89); 61% were male. The most common reasons for initiating treatment at diagnosis were constitutional symptoms (58%), cytopenias (72%; anemia [69%]), and IgM-related symptoms (57%). Choice of therapy varied with line of treatment; monotherapy fell from 31% in front-line to 20%/21% in 2nd/3rd-line (Table 1). Combination therapy with antibody increased from 40% in front-line to 64%/56% in 2nd/3rd-line. Across all lines, rituximab followed by cyclophosphamide, and to a lesser extent, chlorambucil, fludarabine, vincristine, and bendamustine, were the most common agents, excluding steroids, that were used as monotherapy or in any combination with use varying between countries (Table 1). Median PFS decreased with successive lines of treatment (29 vs 23 vs 16 mo), (Figure 1) and varied by country and choice of agents (Table 1).Median OS was 123 mo, but significantly lower in pts ≥75 yr (75 mo) or with high-risk IPSSWM risk score (91 mo) and similar for pts with low/intermediate risk groups. Considerable country-specific OS differences were noted. Other malignancies were reported in 12% after diagnosis of WM.
Conclusions
The retrospective chart review of WM pts treated in Europe shows that constitutional symptoms and anemia are the most common reasons for initiating therapy. Rituximab was the most commonly used agent across all lines of treatment. Outside clinical trials, monotherapy is widely used even at first relapse with notable differences between countries. This large observational dataset will be an important tool to improve understanding of treatment practice and survival of WM pts in Europe outside of clinical trials, as well as unmet medical needs in the community.
Table 1. Use of Monotherapy or Combination Regimens and Median PFS in Front-, 2nd-, and 3rd-Line Settings Overall and by Country
|
Country |
Number of Cases, n |
Monotherapy, % |
Combination Therapy With Antibody†, % |
Combination Therapy Without Antibody, % |
Median PFS, Months (95% CI) |
|||||||||||
|
Front |
2nd |
3rd |
Front |
2nd |
3rd |
Front |
2nd |
3rd |
Front |
2nd |
3rd |
Front |
2nd |
3rd |
||
|
Overall |
454 |
397 |
160 |
31 |
20 |
21 |
40 |
63 |
56 |
28 |
14 |
21 |
29.0 |
23.0 |
16.0 |
|
|
France |
92 |
86 |
43 |
62 |
26 |
16 |
24 |
66 |
70 |
14 |
8 |
14 |
28.5 |
30.0 |
16.0 |
|
|
United Kingdom |
72 |
64 |
19 |
18 |
22 |
21 |
19 |
55 |
42 |
63 |
23 |
37 |
31.5 |
20.0 |
13.0 |
|
|
Germany |
66 |
52 |
18 |
9 |
8 |
22 |
61 |
81 |
50 |
30 |
8 |
11 |
36.5 |
24.0 |
8.0 |
|
|
Spain |
60 |
58 |
21 |
43 |
28 |
38 |
38 |
59 |
52 |
18 |
12 |
5 |
18.0 |
16.0 |
11.0 |
|
|
Italy |
56 |
47 |
20 |
20 |
17 |
15 |
57 |
68 |
70 |
23 |
6 |
15 |
30.5 |
30.0 |
17.0 |
|
|
Eastern European* |
37 |
30 |
12 |
8 |
13 |
8 |
32 |
40 |
0 |
60 |
47 |
92 |
33.0 |
20.0 |
20.5 |
|
|
Smaller European** |
71 |
60 |
27 |
35 |
22 |
26 |
56 |
67 |
63 |
7 |
12 |
11 |
23.0 |
16.0 |
16.0 |
|
*Includes Czech Republic and Poland
**Includes Austria, Greece, and Netherlands
†Antibodies other than rituximab, <1%
Figure 1. Kaplan-Meier PFS Estimates by Line of Treatment
Disclosures: Buske: CELLTRION, Inc.: Consultancy , Honoraria . Sadullah: Roche: Honoraria , Speakers Bureau ;Novartis: Honoraria , Speakers Bureau ; Takeda: Consultancy , Honoraria , Speakers Bureau ; NAPP: Consultancy , Honoraria , Other: Travel, Accommodations, Expenses ; TEVA: Consultancy ; Boehringer: Other: Travel, Accommodations, Expenses . Kastritis: Janssen: Consultancy , Other: Travel, Accommodations, Expenses .Garcia-Sanz: Janssen: Honoraria , Other: Travel, Accommodations, Expenses ; Takeda: Honoraria , Other: Travel, Accommodations, Expenses ; Novartis: Research Funding . Leleu: Pierre Fabre: Honoraria ; BMS: Honoraria ;Novartis: Honoraria ; TEVA: Honoraria ; Amgen: Honoraria ; Takeda: Honoraria ; Celgene: Honoraria ; Janssen:Honoraria ; LeoPharma: Honoraria ; Chugai: Honoraria . Willenbacher: Celgene: Consultancy , Honoraria , Other: Travel, Accommodations, Expenses , Research Funding ; Roche: Consultancy , Other: Travel, Accommodations, Expenses , Research Funding ; Janssen: Consultancy , Other: Travel, Accommodations, Expenses , Research Funding ; Amgen: Consultancy , Other: Travel, Accommodations, Expenses , Research Funding ; Gilead:Consultancy , Other: Travel, Accommodations, Expenses , Speakers Bureau ; Novartis: Consultancy , Honoraria , Other: Travel, Accommodations, Expenses , Research Funding ; CTI: Consultancy , Other: Travel, Accommodations, Expenses . Hajek: Amgen: Honoraria ; Celgene: Consultancy ; Janssen: Consultancy . Cheng:Pharmacyclics LLC, an AbbVie Company: Employment . Graef: Pharmacyclics LLC, an AbbVie Company:Employment , Membership on an entity’s Board of Directors or advisory committees ; AbbVie: Equity Ownership .Dimopoulos: Janssen: Consultancy , Honoraria ; Celgene: Consultancy , Honoraria ; Onyx: Consultancy , Honoraria ; Amgen: Consultancy , Honoraria ; Novartis: Consultancy , Honoraria ; Genesis Pharma: Research Funding .
Datum přednesení příspěvku: 5. 12. 2015