Konference: 2015 57th ASH Annual Meeting - účast ČR
Kategorie: Mnohočetný myelom
Téma: 653. Myeloma: Therapy, excluding Transplantation: Advances in Newly Diagnosed and Relapsed Myeloma
Číslo abstraktu: 30
Autoři: Wee Joo Chng; Prof. Dr. med. Hartmut Goldschmidt; Meletios Athanasios Dimopoulos, MD; Prof. MD Philippe Moreau; Douglas E. Joshua; MD Antonio P. Palumbo; MD Thierry Facon; Prof. MD Heinz Ludwig; prof. MUDr. Luděk Pour, Ph.D.; MD Ruben Niesvizky; M.D. Albert Oriol; MD Laura Rosiñol, PhD; M.D. Alexander Suvorov; MD Gianluca Gaidano, PhD; MUDr. Tomáš Pika; MD Katja C. Weisel; MD Vesselina Goranova-Marinova, PhD; Heidi H. Gillenwater; Nehal Mohamed; Shibao Feng; prof. MUDr. Roman Hájek, CSc.
Introduction: Single-agent carfilzomib has previously shown activity in patients with relapsed and refractory multiple myeloma (MM) who have high-risk cytogenetic abnormalities (Jakubowiak et al, Leukemia 2013;27:2351–56). In the randomized phase 3 study ENDEAVOR (NCT01568866; N=929), carfilzomib plus dexamethasone (Kd) demonstrated a clinically meaningful and statistically significant 2-fold improvement in median progression-free survival (PFS) compared with bortezomib plus dexamethasone (Vd; 18.7 vs 9.4 months; hazard ratio [HR]: 0.53; 95% confidence interval [CI]: 0.44–0.65; P<.0001) (Dimopoulos et al, J Clin Oncol 2015;33:abstr 8509; Dimopoulos et al,Haematologica 2015;100[s1]:abstr LB2071). Herein we present results of a preplanned subgroup analysis of the efficacy and safety of Kd vs Vd in the ENDEAVOR study based on baseline cytogenetic risk status.
Methods: Adult patients with relapsed MM (RMM; 1–3 prior lines of therapy) were eligible. Patients in the Kd arm received carfilzomib (30-minute intravenous [IV] infusion) on days 1, 2, 8, 9, 15, and 16 (20 mg/m2 on days 1 and 2 of cycle 1; 56 mg/m2 thereafter) and dexamethasone 20 mg on days 1, 2, 8, 9, 15, 16, 22, and 23 of a 28-day cycle. Patients in the Vd arm received bortezomib 1.3 mg/m2 (IV bolus or subcutaneous injection) on days 1, 4, 8, and 11 and dexamethasone 20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12 of a 21-day cycle. Cycles were repeated until disease progression, withdrawal of consent, or unacceptable toxicity. The primary end point was PFS. Secondary end points included overall survival, overall response rate (ORR), duration of response (DOR), rate of grade ≥2 peripheral neuropathy (PN), and safety. Fluorescence in situ hybridization was used to assess cytogenetic risk status. The high-risk group was defined as those patients with the genetic subtypes t(4;14) or t(14;16) in ≥10% of screened plasma cells, or deletion 17p in ≥20% of screened plasma cells based on central review of bone marrow samples obtained at study entry; the standard-risk group consisted of patients without these genetic subtypes.
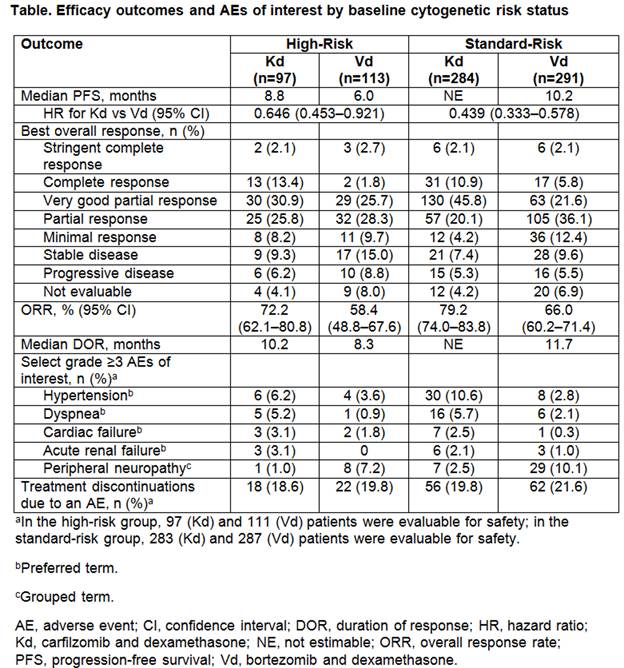
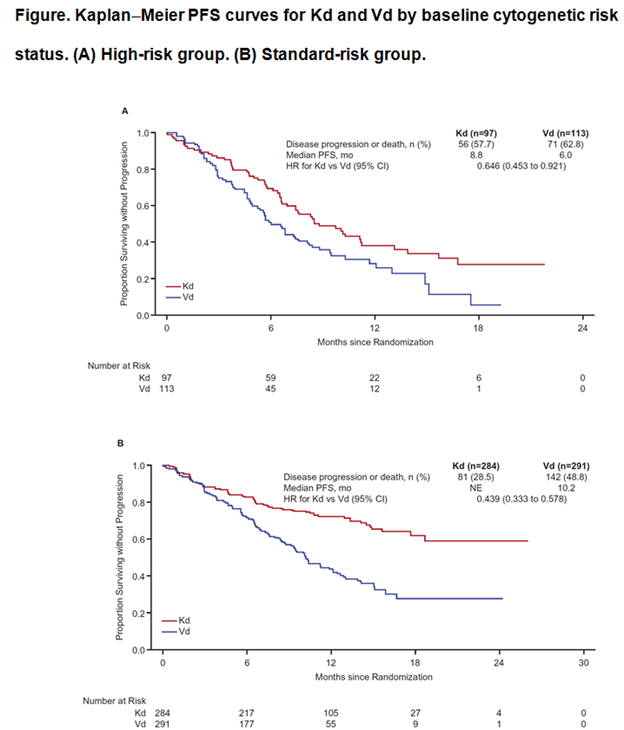
Results: A total of 929 patients were randomized (Kd: 464; Vd: 465). Baseline cytogenetic risk status was well-balanced between the treatment arms (high-risk: Kd, 20.9%; Vd, 24.3%; standard-risk: Kd, 61.2%; Vd, 62.6%; unknown: Kd, 17.9%; Vd, 13.1%). Efficacy end points by baseline cytogenetic risk status are presented in the Table; Kaplan-Meier PFS curves by baseline cytogenetic risk status are shown in the Figure. Median PFS in the high-risk group (n=210) was 8.8 months (95% CI: 6.9–11.3) for Kd vs 6.0 months (95% CI: 4.9–8.1) for Vd (HR: 0.646; 95% CI: 0.453–0.921). Median PFS in the standard-risk group (n=575) was not estimable for Kd (95% CI: 18.7–not estimable) vs 10.2 months (95% CI: 9.3–12.2) for Vd (HR: 0.439; 95% CI: 0.333–0.578). ORRs (≥partial response) were 72.2% (Kd) vs 58.4% (Vd) in the high-risk group and 79.2% (Kd) vs 66.0% (Vd) in the standard-risk group. In the high-risk group, 15.5% (Kd) vs 4.4% (Vd) achieved a complete response (CR) or better. In the standard-risk group, 13.0% (Kd) vs 7.9% (Vd) achieved ≥CR. Median DOR in the high-risk group was 10.2 months for Kd vs 8.3 months for Vd. Median DOR in the standard-risk group was not estimable for Kd vs 11.7 months for Vd. Grade ≥3 adverse events (AEs) were reported at higher rates with Kd vs Vd in the high- and standard-risk groups (70.1% vs 63.1% and 73.9% vs 68.3%). Rates of grade ≥3 AEs of interest by baseline cytogenetic risk status are shown in the Table. Grade ≥2 PN was reported at lower rates with Kd vs Vd in the high-risk group (3.1% vs 35.1%; odds ratio: 0.059; 95% CI: 0.018–0.198) and also in the standard-risk group (6.4% vs 33.4%; odds ratio: 0.135; 95% CI: 0.079–0.231).
Conclusion: As expected, median PFS for patients with high-risk cytogenetics was lower compared with the overall population; however, patients treated with Kd had a clinically meaningful improvement in PFS compared with Vd in patients with high- or standard-risk cytogenetics. Higher response rates, a greater depth of response, and longer DOR were also observed with Kd vs Vd across cytogenetic subgroups. Kd had a favorable benefit–risk profile in patients with high-risk relapsed MM, and was superior to Vd, regardless of baseline cytogenetic risk status.
Disclosures: Goldschmidt: BMS: Consultancy , Research Funding ; Amgen, Takeda: Consultancy ; Onyx:Consultancy , Honoraria ; Janssen, Celgene, Novartis: Consultancy , Honoraria , Research Funding ; Chugai, Millennium: Honoraria , Research Funding . Dimopoulos: Onyx: Honoraria ; Janssen: Honoraria ; Celgene:Honoraria ; Janssen-Cilag: Honoraria ; Genesis: Honoraria ; Amgen: Honoraria ; Novartis: Honoraria . Moreau:Novartis, Janssen, Celgene, Millennium, Onyx Pharmaceuticals: Consultancy , Honoraria . Joshua: Celgene:Membership on an entity’s Board of Directors or advisory committees . Palumbo: Novartis, Sanofi Aventis:Honoraria ; Celgene, Millennium Pharmaceuticals, Amgen, Bristol-Myers Squibb, Genmab, Janssen-Cilag, Onyx Pharmaceuticals: Consultancy , Honoraria . Facon: Onyx/Amgen: Membership on an entity’s Board of Directors or advisory committees . Ludwig: Celgene Corporation: Honoraria , Speakers Bureau ; Onyx: Honoraria , Speakers Bureau ; Bristol Myers Squibb: Honoraria , Speakers Bureau ; Janssen Cilag: Honoraria , Speakers Bureau ; Takeda:Research Funding . Niesvizky: Celgene, Millennium, Onyx: Consultancy , Speakers Bureau . Oriol: Celgene, Janssen, Amgen: Consultancy , Speakers Bureau . Rosinol: Celgene, Janssen: Honoraria . Gaidano: Morphosys, Roche, Novartis, GlaxoSmith Kline, Amgen, Janssen, Karyopharm: Honoraria , Other: Advisory Boards ; Celgene:Research Funding . Weisel: Takeda: Other: Travel Support ; Novartis: Other: Travel Support ; Onyx: Consultancy , Honoraria ; Janssen: Consultancy , Honoraria , Other: Travel Support , Research Funding ; Amgen: Consultancy , Honoraria , Other: Travel Support ; Celgene: Consultancy , Honoraria , Other: Travel Support , Research Funding ;Bristol Myers Squibb: Consultancy , Honoraria , Other: Travel Support ; Noxxon: Consultancy . Gillenwater: Onyx, Amgen: Employment , Other: Stock . Mohamed: Onyx/Amgen: Employment , Other: Stock . Feng: Amgen/Onyx:Employment , Equity Ownership . Hájek: Janssen-Cilag: Honoraria ; Celgene, Amgen: Consultancy , Honoraria .
Datum přednesení příspěvku: 5. 12. 2015