Konference: 2005 XII. Jihočeské onkologické dny
Kategorie: zhoubné nádory mozku a CNS
Téma: Konference bez tematických celků
Číslo abstraktu: 017
Autoři: prof. MUDr. Karel Odrážka, Ph.D.; prof. MUDr. Jiří Petera, Ph.D.; Ing. Milan Zouhar; Pavel Dvorak; MUDr. Tereza Kohlová; prof. MUDr. Martin Doležel, Ph.D.; doc. MUDr. Milan Vošmik, Ph.D.; MUDr. Miloslava Vaculíková; MUDr. Vladimir Hobza, Ph.D.; prof. MUDr. Svatopluk Řehák, CSc.
původní název: COMPARISON OF IMRT AND 3D-CRT TREATMENT
TECHNIQUES
Supported by the Grants NR/8061 from the Internal Grant Agency of
the Ministry of Health, Czech Republic, and 137/2004 C from the
Grant Agency of Charles University in Prague, Czech Republic.
INTRODUCTION
Intensity-modulated radiation therapy (IMRT) offers the potential
of highly conformal dose distribution that allowed improved sparing
of healthy tissues. Contrary to the three-dimensional conformal
radiation therapy (3D-CRT), IMRT is able to combine both spatial
beam shaping and fluence modulation across the beam. Severe late
toxicity after curative radiotherapy for low-grade gliomas has been
observed even with recommended doses ranging from 45 Gy to 55 Gy.
Although the incidence of brain necrosis is rather low, it cannot
be regarded insignificant.
The aim of this study was to compare target volume coverage and
normal tissue sparing of the IMRT and 3D-CRT techniques in the
treatment of low-grade gliomas.
METHODS AND MATERIALS
Patients and target volumes
Ten patients with supratentorial low-grade glioma were selected for
this planning study. The main aim of this research was to determine
the possibility of brain tissue sparing with different treatment
techniques. That is why the patients with tumors in close proximity
to the eyes (anterobasal parts of frontal lobes) were not included
in order to avoid the necessity of sparing other critical
structures beyond brain. Postoperative radiotherapy was given to
three patients while seven patients were primarily treated with
radiotherapy following stereotactic biopsy of the tumor.
The gross tumor volume (GTV) included the tumor volume as defined
by magnetic resonance (MR) scan. In patients treated
postoperatively, the preoperative tumor volume was delineated. A
uniform margin of 15 mm was added to the GTV using an automated
volume expansion to create the clinical target volume (CTV).
Another 5 mm margin was supplemented to the CTV to define the
planning target volume (PTV). Finally, the PTV contour was adjusted
where necessary in order to conform the PTV to the natural barriers
of tumor spread (bones of skull, tentorium).
Radiotherapy planning
Treatment plans were generated using a 3D treatment planning system
(CadPlan R.6.3.6., Varian Medical Systems, Inc., Palo Alto, CA)
with 6 MV photon beams. All patients were planned and irradiated
with a 3-field 3D-CRT technique. Beam arrangements consisted of two
wedged opposed fields (90°, 270°) and a parietal field with
variable angle, ranging from 35° to 90° according to the tumor
location. The PTV was then displayed using beam(s eye view (BEV)
mode and the individual beams were shaped with a multileaf
collimator (MLC). A margin of 8 mm was left between PTV and the
leaves of MLC accounting for beam penumbra.
Another two IMRT plans were created for each patient in this
planning study. A 3-field IMRT plan (IMRT 3F) had a beam
arrangement equal to the 3D-CRT plan. A 5-field IMRT plan (IMRT 5F)
used the very same three fields as a basic set-up. Two additional
fields were added on both sides between the lateral and parietal
fields. These oblique fields were angled (table rotation +45° and
-45°, appropriate gantry rotation) so that the central axes of all
five beams were coplanar. The inverse treatment planning was
performed using the Helios module, which is an integral part of our
3D treatment planning system. A single constraint was defined for
the PTV 100% of the volume should receive a minimum dose of 51.3
Gy, corresponding to 95% of the prescribed dose. This requirement
was weighted with maximal priority (100). No attempt was made to
constrain the dose to surrounding brain tissue.
Treatment plan optimization
Both 3D-CRT and IMRT plans were normalized to the ICRU point
(isocenter). A total dose of 54 Gy in 30 fractions was prescribed
to the ICRU point. The IMRT fields were delivered with a dynamic
multileaf collimator (2x26 leaves) using the sliding window
technique. For valid comparison of the plans, the basic
optimization condition was to cover the entire PTV with 95%
isodose. Moreover, the maximum dose should not exceed 107% of the
prescribed dose.
Comparison of treatment plans
Overall, three plans were produced for a particular patient (Figure
1). Dose-volume histograms (DVH) were generated for the PTV and
brain. Nothing but brain tissue outside PTV was considered for the
DVH calculation. Thus the PTV was subtracted from the brain volume.
The percentage of the PTV covered by 95% isodose was registered in
order to assess the homogeneity of dose distribution. Conformity
index (CI) was calculated according to the equation described by
the ICRU Report 62. The volume of the 95% isodose was regarded as
the treated volume (CI = VPTV/V95%). The volumes of brain (BV)
irradiated to a dose of 10 Gy (BV10), 20 Gy (BV20), 30 Gy (BV30),
40 Gy (BV40), and 50 Gy (BV50) were recorded. Plans were compared
using mean dose-volume statistics. A two-tailed Student’s t-test
was used to detect the differences between treatment plans.
Differences were reported as statistically significant with a p
value less than 0.05. To quantify the fraction of brain encompassed
by the therapeutic 95% isodose volume, the normal tissue-sparing
index (NTSI) was calculated (Miften MM, et al. J Appl Clin Med Phys
2004;5:1-13). Finally, the Lyman model was used to calculate the
normal tissue complication probability (NTCP) and to project the
risks of brain damage. Following parameters were entered for the
calculation: TD50 = 60 Gy, m = 0.15, n = 0.25. The fraction of
irradiated brain volume was determined by the method of effective
volume as proposed by Kutcher and Burman.
RESULTS
Both 3D-CRT and IMRT plans showed an excellent coverage of the PTV.
As much as 99.9%, 100%, and 100% of the PTV was covered by the 95%
isodose for the 3D-CRT, IMRT 3F, and IMRT 5F plans, respectively
(Figure 2). The maximum PTV dose did not exceed 107% in any
treatment plan. The mean CI of the IMRT 5F plan was the highest
(0.71+0.01) followed by the IMRT 3F plan (0.67+0.04) and 3D-CRT
plan (0.59+0.03).
Brain tissue was significantly spared with both IMRT techniques
compared to the 3D-CRT technique (Figure 3, table 1). The volume of
normal brain tissue irradiated to doses of 10 Gy, 20 Gy, 30 Gy, 40
Gy, and 50 Gy was significantly lower for the IMRT 3F plan compared
to the 3D-CRT plan. At the dose level of 40 Gy, the IMRT 3F
technique was associated with 24.1% reduction of the irradiated
brain volume.
No difference has been observed between the IMRT 5F and 3D-CRT
plans at lower doses up to 20 Gy. Starting with the 30 Gy dose
level, the IMRT 5F technique resulted in substantial brain sparing
compared to the 3D-CRT technique. As much as 40.8% reduction of the
brain volume receiving dose of 50 Gy was recorded for the IMRT 5F
plan. Intensity modulation enabled to decrease the undesirable dose
delivered to the brain tissue.
The mean NTSI of the IMRT 5F plan was the highest (0.79+0.07)
followed by the IMRT 3F plan (0.75+0.07) and 3D-CRT plan
(0.64+0.11). According to the NTCP model calculation, the predicted
hazard of brain necrosis was approximately 2.0% after 3D-CRT
treatment. By contrast, this was only 1.0% and 0.6% for the IMRT 3F
and IMRT 5F techniques, respectively.
CONCLUSION
Relatively simple IMRT techniques with coplanar beam arrangement
showed a significant brain sparing in comparison with the 3D-CRT
technique. Regarding the development of late toxicity, IMRT may be
beneficial for the longterm survivors with low-grade gliomas.
Figure 1 Dose distribution on frontal plane
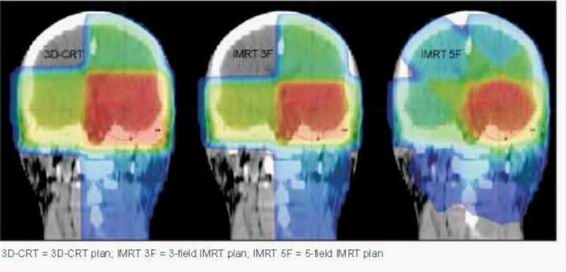

3D-CRT = 3D-CRT plan; IMRT 3F = 3-field IMRT plan; IMRT 5F =
5-field IMRT plan; SD = standard deviation; BV10-50 = percentage of
the brain volume receiving a dose of 10 Gy, 20 Gy, 30 Gy, 40 Gy,
and 50 Gy, respectively.
The p values denote statistical significance of the two-tailed
Student’s t-test comparison of the treatment plans: 3D-CRT vs. IMRT
3F*, 3DCRT vs. IMRT 5F**, and IMRT 3F vs. IMRT 5F***.
Datum přednesení příspěvku: 14. 10. 2005





