Acute renal failure (ARF) is a severe complication of MM often
leading to permanent renal dysfunction and dependence on chronic
hemodialysis. Reversal of kidney failure can only be achieved by
fast and substantial suppression of pathogenic light-chains by
effective anti-MM therapy. In a previous pilot study we were able
to reverse renal impairment with bortezomib based therapy in 5 of 9
pts (Haematologica 2007). Here, we present preliminary data from an
ongoing phase II study in pts with ARF using the BDD regimen. Up to
now 37 pts with MM induced ARF (age: median 64 yrs, range 41-82
yrs, DS stage I: 13%, II: 10%, III: 77%; IgG: 18%, IgG: 23%; Light
chain: 32%, Light chain: 27%) have been enrolled. Seventeen (46%)
pts presented with de novo MM, and 20 with progressive disease. ARF
was defined as reduction of GFR to <50ml/min due to MM
nephropathy in newly diagnosed pts, and as reduction of GFR by
>25% and to <60ml/min in pts with previously treated MM and
GFR of >60ml/min within the last 4 weeks and with signs of tumor
progression. Treatment regimen: Bortezomib 1.0mg/m2, d1,4,8,11,
doxorubicin 9mg/m2, d1,4,8,11 until first safety analysis after
enrollment of the first 5 pts and thereafter of 9mg2, d1,4, and
dexamethasone 40mg d1,4,8,11. Cycles were repeated every 21 days.
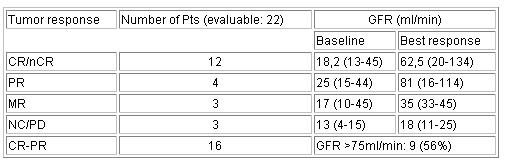
22 pts have completed at least 3 cycles and are evaluable for
response as yet. Overall response rate was 73% including 8 pts
achieving CR, 4 nCR, 4 PR; 3 pts achieved MR. Median GFR at
baseline was 17ml/min (range: 4 - 45ml/min) and improved to 45,5
ml/min (range: 11 - 134ml/min). A significant increase in GFR
(>75ml/min) was achieved in 9 of the 16 pts with CR-PR while in
pts with MR or NC/PD no improvement in GFR was seen. Toxicity was
assessed in 25 pts including 3 pts not evaluable for response.
Grade 1-2 toxicities (>10% of pts): anemia 40%, neutropenia 23%,
thrombopenia 27%, fatigue 50%, infections/fever 64%, neuropathy
36%, edema 32%; diarrhea 27%, nausea 27%, mucositis 23%; Grade 3-4
toxicities: anemia 9%, neutropenia 23%, thrombopenia 9%, infections
18%, neuropathy 1%. Three of the infectious complications were due
to herpes virus infections/reactivations. Three pts died during the
first treatment cycle; 2 pts from pneumonia (including 1 with
sepsis) and 1 pt (age 81 yrs) from myocardial infarction. This led
to an adaptation of the treatment regimen including a reduction in
the frequency of doxorubicin administration to days 1 and 4
(instead of d1,4,8,11), abandonment of the planned dose increase of
bortezomib (to 1.3mg/m2), and addition of mandatory antibacterial
and antiviral prophylaxis. In conclusion, overall anti-myeloma
response rate in the 22 evaluable pts was 73%, with 12 (54%) pts
achieving CR/nCR; ARF could be reverted in 9 pts. (41% of total or
56% of pts. with CR-PR). After dose reduction of the initial
regimen, treatment was well tolerated in this high-risk and often
multimorbid patient population.
Abstract #3603 appears in Blood, Volume 110, issue 11, November 16,
2007
Keywords: Clinical Trial|Free Light Chain|Remission
Disclosure: Research Funding: Schering-Plough, Janssen-Cilag.
Honoraria Information: Janssen-Cilag, Roche, Amgen, Celgene.
Off Label Use: Bortezomib in newly diagnosed patients with acute
renal failure.
Monday, December 10, 2007 5:00 PM
Session Info: Poster Session: Myeloma: Novel Therapies (5:00
p.m.-7:00 p.m.)






