Konference: 2013 18th Congress of the European Hematology Association - účast ČR
Kategorie: Mnohočetný myelom
Téma: Clinical studies in Multiple Myeloma
Číslo abstraktu: S1153
Autoři: MD Sara Bringhen; Prof. Dr. S. Vincent Rajkumar; Giulia Lupparelli, PhD; Dr. Saad Zafar Usmani; Prof. Anders Waage; MD Alessandra Larocca; Bronno van der Holt; MD Pellegrino Musto; Andrea Evangelista, PhD; MD Sonja Zweegman, PhD; Meletios Athanasios Dimopoulos, MD; prof. MUDr. Roman Hájek, CSc.; MD Michele Cavo; Prof. MD Sagar Lonial; MD Giovannino Ciccone, PhD; MD Mario Boccadoro; Prof. MD Bart Barlogie, PhD; MD Pieter Sonneveld, PhD.; Philip L. McCarthy, BA, MD; MD Antonio P. Palumbo
Background:
Three large randomized trials recently reported an increased risk of second primary malignancies (SPMs) in newly diagnosed myeloma (MM) patients treated with lenalidomide. However, patients with MM have multiple risk factors for SPM and the excess risk with lenalidomide compared with non-lenalidomide has not been described.
Aims:
We performed an individual patient data meta-analysis to estimate the incidence of SPM according to lenalidomide exposure.
Methods:
Relevant studies, from PubMed and ASCO/IMW/ASH abstracts (after 2000), that met the following criteria were included: (1) randomized trials of newly diagnosed MM patients; (2) randomization to treatment with lenalidomide in at least one arm (lenalidomide-trials); (3) randomization to treatment to at least one new drug but not lenalidomide (no-lenalidomide-trials); (4) available data of SPMs. The primary aim was the cumulative incidence of SPMs, that was estimated accounting for competing events (Gooley at al).
Results:
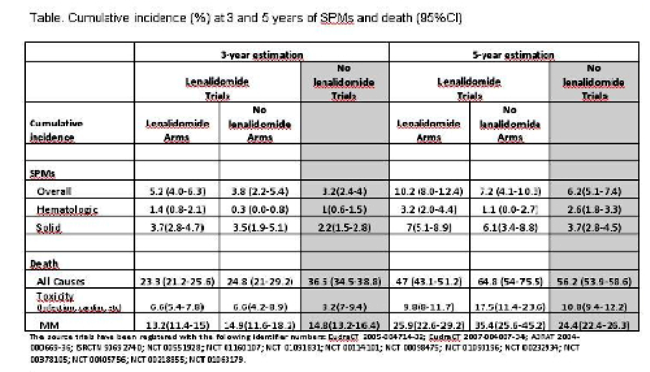
6383 patients were included in the analysis. The median follow-up was 30 months. Median age was 69 years, 45% of patients aged 65-74 years and 22% ≥75 years. Total cases of SPMs were 420 (6.6%), including 188 (2.9%) hematologic and 232 (3.6%) solid cancers. The cumulative incidence at 3 and 5 years of SPMs and of death were summarized in the table. 3218 patients were enrolled in the lenalidomide-trials and were available for a direct comparisons between lenalidomide vs non-lenalidomide treatments. Solid tumors occurred with similar incidence in all treatment groups. The risk of hematologic SPM was significantly higher in patients receiving lenalidomide (5-year cumulative incidence 3.2% vs 1.1%, p=0.04) and increased linearly over time. The risk is limited to patients receiving lenalidomide plus melphalan (4.1, 95%CI: 2.4-5.8) with no excess in other combinations (lenalidomide without melphalan: 1.2, 0.0-2.6; melphalan without lenalidomide: 1.1, 0.0-2.7) (p=0.003). The risk of death for adverse events and for progression was higher than the risk of SPM.
Image / Pictures:
Summary / Conclusion:
An increase of SPMs was observed in patients receiving lenalidomide compared with controls. The observed difference was attributed to the increased occurrence of hematologic SPMs, mainly with combination including lenalidomide plus melphalan. In the context of the observed survival benefit, the benefit/risk profile of lenalidomide treatment remains positive.
Datum přednesení příspěvku: 16. 6. 2013