Konference: 2015 57th ASH Annual Meeting - účast ČR
Kategorie: Nádorová biologie/imunologie/genetika a buněčná terapie
Téma: 641. CLL: Biology and Pathophysiology, excluding Therapy: Poster
Číslo abstraktu: 1712
Autoři: Mgr. Veronika Navrkalová, Ph.D.; Emma Young; Panagiotis Baliakas; Mgr. Lenka Radová, Ph.D.; Mgr. Karla Plevová, Ph.D.; Lesley-Ann Sutton; Larry Mansouri; Viktor Ljungström; Stavroula Ntoufa; Zadie Davis; Prof. MD Gunnar Juliusson, PhD; M.D. Karin Ekström Smedby, Ph.D.; MD Chrysoula Belessi; M.D. Panagiotis Panagiotidis; MD Frederic Davi, PhD; Dr. Anton W. (Ton) Langerak ; M.D. Paolo Ghia, Ph.D.; BSc. Jonathan C. Strefford, PhD; Prof. David Graham Oscier; MD Kostas Stamatopoulos; prof. RNDr. Šárka Pospíšilová, Ph.D.; Prof. MD Richard Rosenquist (Brandell), PhD; Doc. MUDr. Martin Trbušek, PhD
Chronic lymphocytic leukemia (CLL) is a paradigmatic malignancy where the interplay of cell-extrinsic and cell-intrinsic factors has a major impact on disease evolution. Indeed, extrinsic triggering, e.g. antigenic stimulation through the B-cell receptor (BcR), together with intrinsic aberrations, e.g. accumulation of genetic defects, play a major role throughout the natural history of CLL. The importance of antigen involvement is underscored by the existence of ‘stereotyped’ BcR in up to 30% of CLL patients. Notably, CLL patients with stereotyped BcR can be grouped into different subsets, each with a subset-biased biological and clinical profile. For instance, while the clinically aggressive subset #2 (IGHV3-21/IGLV3-21, comprising both mutated (M-CLL) and unmutated (U-CLL) IGHV genes) displays a remarkably high frequency of SF3B1 mutations, subset #8, a subset with the highest risk of Richter transformation, shows a strong association with trisomy 12 and NOTCH1 mutations. ATM defects are implicated in the evolution of CLL and are associated with a dismal prognosis, however the extent to which they contribute to the genetic landscape in stereotyped subsets remains unexplored.
To gain insight into this issue, we assessed the frequency of ATM mutations in 249 well-characterized CLL patients assigned to major subsets #1-8. The entire coding region of ATM (62 exons) was investigated with two different targeted deep-sequencing approaches, i.e. Haloplex technology (HiSeq, coverage ~1500X) or the Nextera XT kit (MiSeq, coverage ~4000X). A conservative variant allele frequency cut-off of 10% was selected, and mutations were validated by Sanger sequencing.
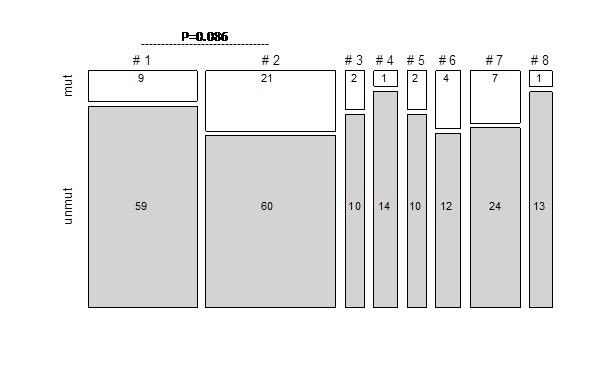
Altogether, we identified 61 ATM mutations in 47/249 (19%) patients across all major subsets (Fig. 1). As expected, the majority of identified ATM mutations (n=43, 70%) have not yet been reported while the remaining 30% were listed in the HGMD or COSMIC mutation databases. The spectrum of ATM mutations included missense (n=31), nonsense (n=9), splicing (n=6), and frame-shift (n=14) mutations, and one in-frame deletion. Missense substitutions were distributed along the entire gene without any ‘hotspot’ region or preferred domain. The highest mutation frequency was detected in subset #2 (26%), with a significant enrichment in U-CLL vs. M-CLL cases, (13/33 vs. 8/48 subset #2 cases, respectively; p=0.021). Within poor-prognostic U-CLL subsets, ATM mutations were also frequent in subsets #6 (25%) and #7 (23%), while subsets #3, #5, #1, and #8 showed lower frequencies (17%, 17%, 13%, and 7%, respectively). The favorable prognostic M-CLL subset #4 exhibited a low frequency (7%) ofATM mutations. Notably, when comparing the two most populated subsets, i.e. #1 and #2, ATM mutations were overrepresented in the latter with a borderline significance value (p=0.086); when restricting the analysis to U-CLL #2 cases a significantly higher frequency was observed compared to #1 (13/33 vs 9/68; p=0.0045).
Regarding the clinical impact of ATM defects in subset #2, we divided patients into subgroups with biallelic inactivation (def-ATM), sole 11q-, sole ATM-mutation, TP53-aberrations and WT. While both groups with mono-allelic ATM disruption showed a significantly reduced overall survival compared to WT (median survival sole ATM-mutation, 71 months, sole 11q-, 40 months, vs. 123 months in the WT group; p=0.002 and 0.02, respectively), a non-significant reduction of overall survival was observed for patients with bi-allelic ATM aberrations (70 months, p=0.29) (Fig. 2). The few subset #2 patients with TP53 defects showed a similar survival as WT group, underscoring previous observations that TP53 dysfunction per se plays a minor role in this subset.
In summary, we demonstrate that ATM mutations can be added to the list of genetic defects with a biased distribution in stereotyped subsets. The enrichment of ATM defects in subset #2 was associated with a negative impact on overall survival, suggesting a role for ATM inactivation in shaping the aggressive phenotype of this subset. This study further supports the recent suggestion that CLL development is driven by antigenic selection, coupled with preferential acquisition of specific genetic defects. The work was supported by the projects MSMT CR CZ.1.05/1.1.00/02.0068, IGA NT13493-4/2012 and TACR TE02000058.
Fig. 1:
Fig. 2:
Disclosures: Langerak: Roche: Other: Lab services in the field of MRD diagnostics provided by Dept of Immunology, Erasmus MC (Rotterdam) ; DAKO: Patents & Royalties: Licensing of IP and Patent on Split-Signal FISH. Royalties for Dept. of Immunology, Erasmus MC, Rotterdam, NL ; InVivoScribe: Patents & Royalties: Licensing of IP and Patent on BIOMED-2-based methods for PCR-based Clonality Diagnostics. . Strefford: Roche: Research Funding . Stamatopoulos: Gilead Sciences: Research Funding ; Janssen Pharmaceuticals: Research Funding .
Datum přednesení příspěvku: 5. 12. 2015